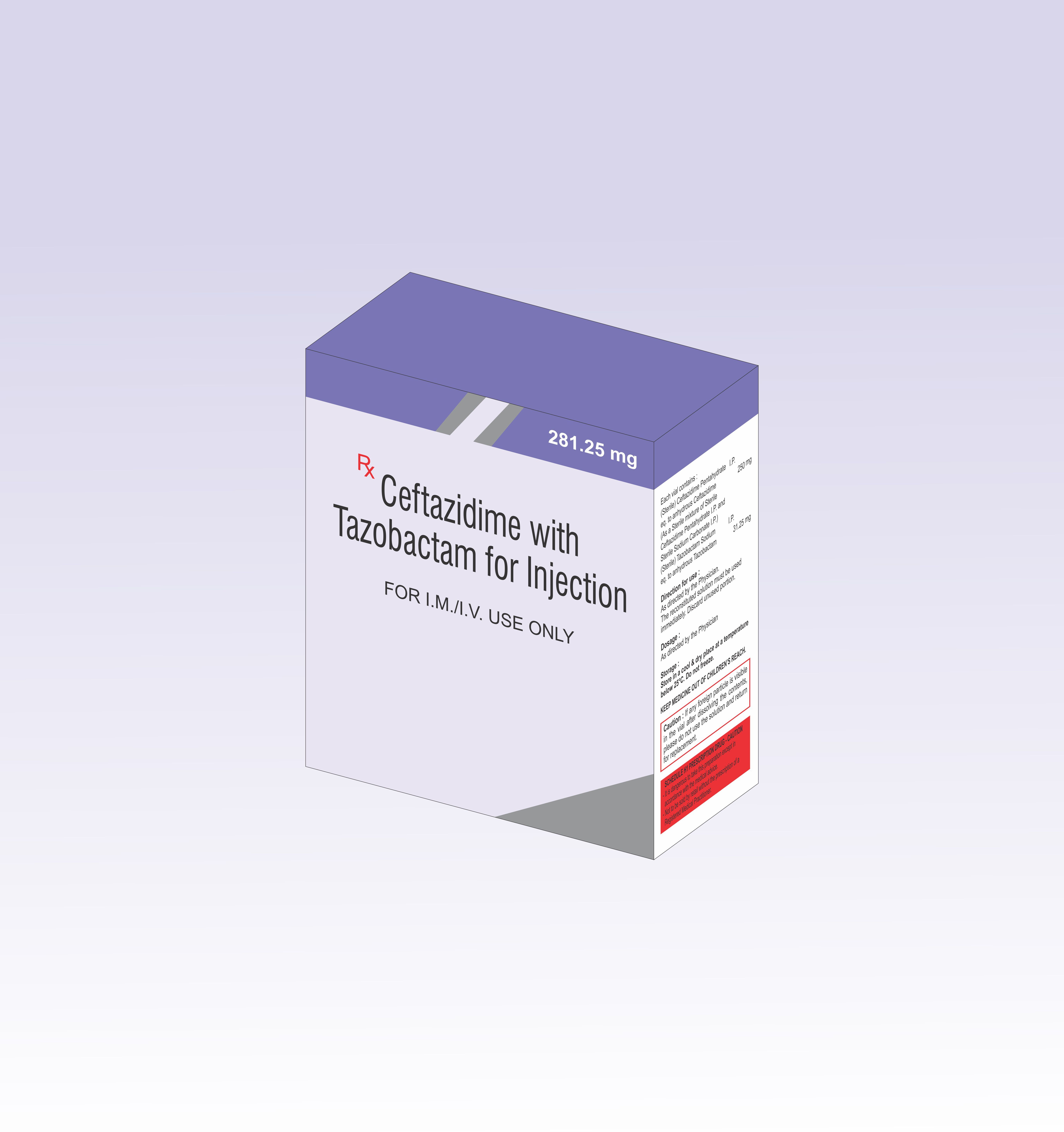
CEFTAZIDIME WITH TAZOBACTAM INJECTION IN THIRD PARTY MANUFACTURING
350 INR/Piece
Product Details:
- Drug Type Injection
- Ingredients Ceftrixone sulbactam 1500 mg
- Physical Form Powder
- Function Antibiotic Medicine
- Recommended For IM/IV USE
- Dosage As Directed by the physician
- Dosage Guidelines As directed by the Physician
- Click to View more
X
CEFTAZIDIME WITH TAZOBACTAM INJECTION IN THIRD PARTY MANUFACTURING Price And Quantity
- 350 INR/Piece
- 3000 Piece
CEFTAZIDIME WITH TAZOBACTAM INJECTION IN THIRD PARTY MANUFACTURING Product Specifications
- As directed by the Physician
- 1000 Boxes
- Antibiotic Medicine
- IM/IV USE
- Powder
- Injection
- STORE IN A COOL & DRY PLACE AT A TEMPERATURE BELOW 25 C. DO NOT FREEZE.
- Ceftrixone sulbactam 1500 mg
- As Directed by the physician
- Suitable For All, Teenagers, Women, Adults, Aged Person
CEFTAZIDIME WITH TAZOBACTAM INJECTION IN THIRD PARTY MANUFACTURING Trade Information
- 100000 Piece Per Month
- 1 Months
- Free samples available with shipping and taxes paid by the buyer
- 20ML VIAL & 30ML VIAL
- Asia
- Assam, Jammu and Kashmir, Tripura, Meghalaya, Pondicherry, Lakshadweep, Gujarat, South India, Central India, North India, East India, West India, Andaman and Nicobar Islands, Andhra Pradesh, Arunachal Pradesh, Bihar, Chandigarh, Delhi, Dadra and Nagar Haveli, Daman and Diu, Goa, Haryana, Himachal Pradesh, Jharkhand, Karnataka, Kerala, Madhya Pradesh, Maharashtra, Mizoram, Manipur, Nagaland, Odisha, Punjab, Rajasthan, Sikkim, Uttarakhand, West Bengal, Uttar Pradesh, Tamil Nadu, Telangana, Chhattisgarh, All India
- GMP
Product Description
We are themanufacturer of Pharmaceutical Injection.Sunvet PharmaPrivateLimited establishedin 2007 with motto to producebestquality products. Since 2007 we have successfullydelivered ourcommitment.Now due to ourexperienceof more than 13 years of Pharmaceutical injection we are thelargest manufacturer of Pharmaceutical injections having following range1. Dry Injections BetaLactam Range(Ceftriaxone Group,Cefoperazone Group with the combination of Sulbactam & Tazobactam,Meropenem injection, Piperacillin Tazobactam Injection and many more)2. Liquid InjectionsRange(Amikacin ,Gentamicin,Methylcobalamin B Complex etc3. Ampoule SectionGeneral Range(OndansetroneInjection, Methylcobalamin with Pyridoxine Hydrochloride, Niacinamide Injection,All range of Methylcobalamin Injection, Piracetam Injection, CiticolineInjection, Diclofenac sodium injection 75 mg/ml Aqua Base, NandroloneDecanoate, etc4.Hormonal range(HydroxyprogesteroneCaproate injection, Progesterone Injection etc5. VeterinaryInjectionBothin Dry and Liquid InjectionNote: Our Veterinary range is very huge and we are specialists of VeterinaryInjections.FAQs of CEFTAZIDIME WITH TAZOBACTAM INJECTION IN THIRD PARTY MANUFACTURING:
Q: What is the recommended storage condition for CEFTAZIDIME WITH TAZOBACTAM INJECTION in third-party manufacturing?
A: The injection should be stored in a cool and dry place at a temperature below 25C. It should not be frozen.Q: What is the physical form of CEFTAZIDIME WITH TAZOBACTAM INJECTION?
A: The product is available in powder form.Q: What type of medicine is CEFTAZIDIME WITH TAZOBACTAM INJECTION?
A: It is an antibiotic medicine.Q: Who is the CEFTAZIDIME WITH TAZOBACTAM INJECTION suitable for?
A: It is suitable for allteenagers, women, adults, and aged persons.Q: How should the dosage of CEFTAZIDIME WITH TAZOBACTAM INJECTION be determined?
A: The dosage should be as directed by the physician.Q: What is the recommended method of administration for CEFTAZIDIME WITH TAZOBACTAM INJECTION?
A: The injection is recommended for IM (intramuscular) or IV (intravenous) use.Q: What quantity is available for third-party manufacturing?
A: The product is available in 1000 boxes.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email